Lesson 007 – Cooling mixture
You need:
Two bowls, waterproof thermometer, salt, iTriangle online, buzzer
|
|
|
||
|
Waterproof thermometer |
Buzzer |
Introduction:
When water changes to a solid as it freezes, its temperature does not change. All heat lost caused by a cooling mixture causes a change of state of the water. Only after the water in the container has solidified will additional heat loss result in a drop in temperature to lower values. In this experiment, the cooling matter (the cooling mixture) removes heat from warmer matter (water). The experiment verifies the understanding that the temperature of the substance does not change during its change of state.
Explanation of coolant temperature (crushed ice + sodium chloride):
Ice melts at 0 °C. The ice-salt mixture has a melting point substantially lower (down to -20 °C). When crushed ice is mixed with sodium chloride, the ice will start melting rapidly because the mixture has a much higher temperature (about 0 °C) at that time than its freezing point. To melt ice in this way (breaking its crystal structure) requires energy (heat) to be removed at the expense of the total temperature of the salt water produced. Salting the crushed ice at a temperature of about 0 °C turns it into salt water whose temperature drops down to between -15 °C and -20 °C. The lowest temperature we can reach is -20 °C. Lower temperatures can not be achieved. For this reason, it does not make sense to sprinkle roads with salt. Practically, however, sodium chloride stops dissolving at temperatures below -8 °C to -10 °C. Defrosting is only effective at these values.
Task:
Create a cooling mixture that can freeze water. This experiment will show you how water behaves when it changes state.
Tutorial:
Step 1:
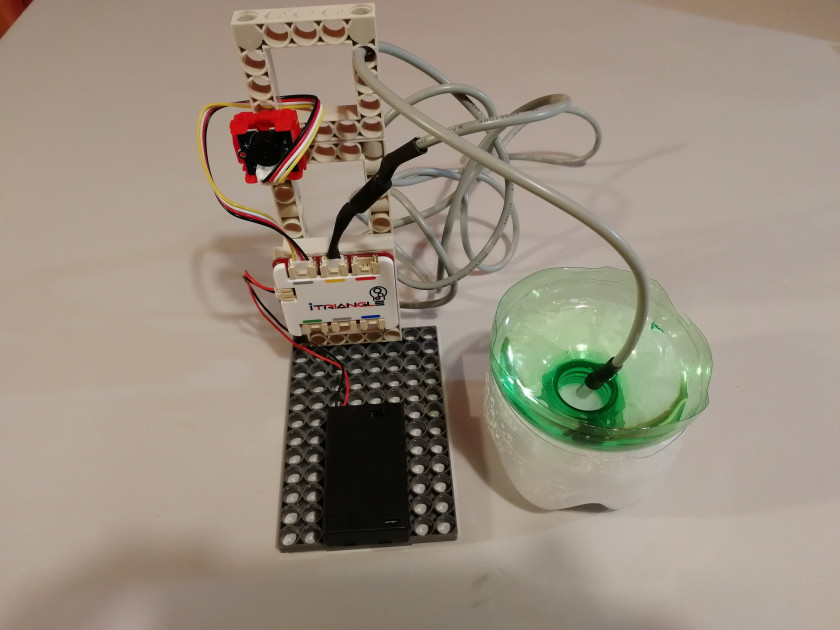
- Construct a simple stand with iTriangle online and connect the sensors and components as pictured below. Connect iTriangle online and upgrade its firmware by following these steps.
- Prepare a mixture of crushed ice and salt (2 parts of ice to 1 part of salt) in a bowl. Mix both together thoroughly and insert the waterproof thermometer into the bowl.
- Start the graphical measurements.
- Watch how the temperature of the mixture falls. When the temperature stops falling, you can stop measuring.
- The temperature of the mixture can fall to as low as -18 °C. To achieve this, you might need to stir the mixture continuously, which you can do with the thermometer directly.
Step 2:
- Re-use the stand from the previous step.
- Prepare a mixture of crushed ice and salt (3 parts of ice to 1 part of salt). Mix both together thoroughly. Take another bowl with water and place it on top of the cooling mixture. Insert the waterproof thermometer into the bowl with water.
- Start the graphical measurements.
- Watch how the temperature of the water falls. When the water turns to ice, you can stop measuring.
- The temperature of the water can fall as low as -12 °C. To achieve this, you might need extra cooling mixture prepared on the side.
Step 3:
- Re-use the stand from the previous steps.
- Prepare a mixture of crushed ice and salt (3 parts of ice to 1 part of salt). Mix both together thoroughly. Take another bowl with water and place it on top of the cooling mixture. Insert the waterproof thermometer into the bowl with water.
- Start the program.
- The program will take measurements 10 times.
- There are two possible outcomes from the measurements.
- If the temperature is higher than 0 °C, the buzzer will produce a short tone
- If the temperature is lower than 0°C, the buzzer will produce a long tone
- Leave the water in the cooling mixture until it becomes solid. If needed, you can start the program again. You also can change the number of measurements to 20 or higher or a lower number.




Žádné komentáře